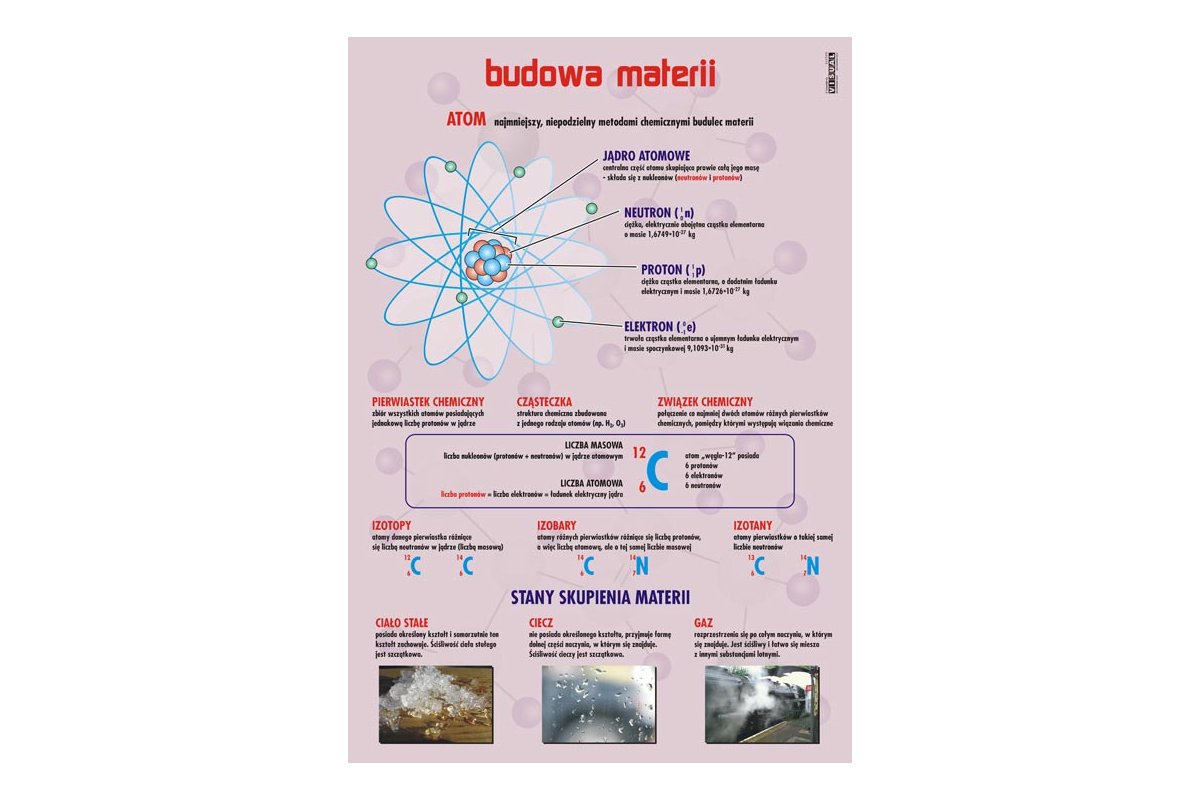
Sprawdzian Z Budowy Wewnętrznej Materii Nowa Era

Sprawdzian Z Budowy Wewnętrznej Materii Nowa Era (Nowa Era's Test on the Internal Structure of Matter) is a subject-specific assessment, typically encountered in Polish secondary schools, focusing on the composition and characteristics of matter at its most fundamental levels. Think of it as a deep dive into atoms, molecules, and the forces that hold them together. This knowledge is crucial for understanding chemistry, physics, and even biology; it underpins fields like materials science, medicine, and energy production.
Solving Problems: A Step-by-Step Guide
When tackling problems in this test, remember these key areas:
- Atomic Structure: Focus on the proton, neutron, and electron. Understand atomic number (liczba atomowa), mass number (liczba masowa), and isotopes.
- Example: Calculate the number of neutrons in Uranium-238. (Answer: Mass number - Atomic number = 238 - 92 = 146)
- Periodic Table: Be familiar with groups (grupy), periods (okresy), metals, non-metals, and metalloids. Recognize the trends in electronegativity, ionization energy, and atomic radius.
- Example: Which element is more electronegative, oxygen or sulfur? (Answer: Oxygen, because electronegativity increases as you move up a group).
- Chemical Bonding: Master ionic (wiązanie jonowe), covalent (wiązanie kowalencyjne - both polar and nonpolar), and metallic bonding (wiązanie metaliczne). Know the conditions under which each forms.
- Example: Explain why sodium chloride (NaCl) has a high melting point. (Answer: Ionic bond, strong electrostatic attraction between ions).
- Molecular Geometry: Learn VSEPR theory to predict the shapes of molecules. Understand the difference between bonding and non-bonding electron pairs.
- Example: What is the shape of a water molecule (H2O)? (Answer: Bent, due to two bonding pairs and two lone pairs on the oxygen atom).
- States of Matter and Phase Changes: Understand the differences between solid, liquid, gas, and plasma. Know the processes of melting, freezing, boiling, condensation, sublimation, and deposition.
- Example: What happens to the kinetic energy of water molecules when water boils? (Answer: Kinetic energy increases).
- Intermolecular Forces: Learn about hydrogen bonding, dipole-dipole interactions, and London dispersion forces. Understand their relative strengths and how they affect physical properties like boiling point.
- Example: Why does water have a relatively high boiling point compared to molecules of similar size? (Answer: Hydrogen bonding).
Quick Fixes: When stuck on a problem, try drawing a diagram, reviewing relevant definitions, or working through similar example problems. Remember to pay attention to the units and use the correct formulas. Understanding the *underlying concepts* is more crucial than memorizing facts.
By focusing on these key areas and practicing regularly, you can confidently tackle Sprawdzian Z Budowy Wewnętrznej Materii Nowa Era.